(右圖為2007年8月27日月全食時的偏食)
2011年12月10日的月全食是很好的教學時機,觀測時間非常恰當,台灣月食時間預測如下:
半影食開始:19:31:54月球與地球半影第一次接觸,必須使用望遠鏡來觀測。
月偏食開始:20:45:24 月球與地球本影第一次接觸,肉眼可見月亮東邊變暗。
月全食開始:22:05:42 月球已完全進入地球本影中,肉眼可見月亮呈現紅色。
月全食結束:22:58:00 月球正要開始離開地球本影,肉眼可見月亮西邊生光。
本影食結束:00:18:18月球正好完全離開地球本影,肉眼可見月亮完全復圓。
半影食結束:01:31:42月球正好完全離開地球半影,必須使用望遠鏡來觀測。
錯過這次的月全食,下一次在台灣要能見到完整的月全食過程是在2018年的1月31日,而最近一次可以看到不完整的月全食(月出帶食)則是在2014年10月8日。
2011/12/10 月全食相關資料:
NASA預測資料:
http://eclipse.gsfc.nasa.gov/LEplot/LEplot2001/LE2011Dec10T.pdf
台北天文台完整資料:
http://tamweb.tam.gov.tw/bew/TW/content.asp?mtype=c9&idx=266
台北天文台月全食動畫與新聞資料下載:
http://tamweb.tam.gov.tw/bew/TW/content.asp?mtype=c9&idx=272
月全食成因動畫:
http://csep10.phys.utk.edu/astr161/lect/time/lunar_anim.html
http://www.teachersdomain.org/asset/psu10phy_vid_eclipser/
月全食教學海報下載:
http://tamweb.tam.gov.tw/bew/TW/content.asp?mtype=c9&idx=273
2011/12/06
2011/11/22
透鏡成像:免費模擬軟體--光學透鏡 (Optical Lenses)
一套好用的透鏡成像模擬軟體,最重要的是:它免費!
只要到下列網址下載:
http://download.cnet.com/Optical-Lenses/3000-2054_4-75416337.html?tag=mncol;7
然後按照指示安裝即可。操作時只要以滑鼠左鍵移動物體,則光線及像會跟著移動,很方便老師做解說。
老師可以用來教:
1.成像作圖;
2.歸納物距與像距、像的大小、和像的性質之間的關係。
你還想到可以教什麼嗎? 你想可以如何用在教學上?
不過要注意提醒學生,物體發出(反射)的光線不是只有那幾條,也不是只有那一點。
只要到下列網址下載:
http://download.cnet.com/Optical-Lenses/3000-2054_4-75416337.html?tag=mncol;7
然後按照指示安裝即可。操作時只要以滑鼠左鍵移動物體,則光線及像會跟著移動,很方便老師做解說。
老師可以用來教:
1.成像作圖;
2.歸納物距與像距、像的大小、和像的性質之間的關係。
你還想到可以教什麼嗎? 你想可以如何用在教學上?
不過要注意提醒學生,物體發出(反射)的光線不是只有那幾條,也不是只有那一點。
靜力平衡: 萬夫莫敵--力的挑戰 (One Man Army: Strength Challenge)
Discovery的"萬夫莫敵(One man army)"競技節目,有一個項目(影片06:56起)很適合用來教靜力平衡。可以先說明比賽規則,請學生想出贏取比賽的方法,然後看影片,再請學生畫力圖分析得勝的原因。裡面牽涉到共點三力平衡、共線二力平衡、及合力概念。
2011/07/20
通往宇宙之窗:美國地球科學教師協會提供的教學資源網站
美國地球科學教師協會(The National Earth Science Teachers Association, NESTA) 開放「通往宇宙之窗(Windows to the Universe)」新網站,提供K-12地球與太空科學教育者教學資源。
「通往宇宙之窗(Windows to the Universe)」網站包含9000頁以上的內容,以及上百個測試過的教學活動,可供教師隨時運用於教學。
美國教師學會(NSTA)也提供「變遷中的地球行星(Our Changing Planet」系列教學活動設計12單元,配合優美動人的「NBC學習(NBC Learn)」影片,是進行環境教育,探討環境及氣候變遷教學的寶庫。
- 通往宇宙之窗(Windows to the Universe)網址:
http://www.windows2universe.org/
- 變遷中的地球行星(Our Changing Planet)網址:
http://www.windows2universe.org/earth/changing_planet/changing_planet.html
2011/04/23
今日化學:分子結構、特性
Chemistry Now: Molecule Structure, Properties
美國科學基金會(NSF)、美國科學教師協會(NSTA)及美國國家廣播公司(NBC)合作,為化學年製作了一系列教學單元,名為「今日化學(Chemistry Now)」,每周推出一單元,預計製作31單元。內容涵蓋日常生活中的化學,包含了21世界的先進化學。以下是第三單元:「鏡」分子--香芹酮("Mirror" Molecule: Carvone)。
旁白:
"Mirror" Molecule: Carvone
Spearmint. Caraway. Dill.
A chewing gum flavor. A seed in rye bread. And an herb in pickles. Wouldnt seem they have anything in common, but they do. Reduce spearmint, caraway seed and dill to their essential oils and a sizable percentage of all three turn out to be made up of the same molecule: carvone.
How can you explain one molecule being responsible for distinctly different smells and tastes? With two hands, a mirror, a lightbulb and a pair of gloves.
Start with the basics: Carvones chemical formula tells you what its made of: 10 atoms of Carbon, 14 atoms of Hydrogen, and 1 atom of Oxygen. Just as important as how many atoms of what elements make up a molecule is how those atoms are bonded together and in what configuration, or structure.
We think of molecules, when and if we think of molecules, as having only one set structure. This is H2O, for example not this, or this. But a molecule like Carvone can have slightly different arrangements of their atoms and still be the same molecule like a girl who has different looks depending on how she parts her hair, or the way she wears a sweater. As long as how she rearranges her hair and clothes doesnt add or take away anything, shes still the same girl in the same outfit.
The carvone molecule has two slightly different looks or, as theyre called, stereoisomers: this one and this one. Stereoisomers are three-dimensional, but its easier to understand these two by looking at a standard two-dimensional drawing of their structures. Notice anything? The two are mirror images of each other. Some structures, like, say, a lightbulb, are the same in mirror image or side by side. But carvone stereoisomers are like a pair of hands: A hand and its mirror reflection will exactly match but hands arent the same side by side, or superimposed.
Actually, carvone stereoisomers are like left and right hands even down to their names: This one is R-carvone the R stands for the Latin word meaning right. The structure of this gives spearmint its taste and smell. Its mirror opposite is S-carvone the S stands for the Latin word meaning left. The way this one is arranged is what gives caraway its flavor and aroma and dill, too. In pure form, the two flavors are almost the same.
This kind of left-handed/right-handed molecule is called chiral. Carvone has chirality, terms, not coincidentally, from a Greek word meaning hand. The Greek word for nose is rhinous, yes, as in rhinocerous. which is the way well segue into this next part on how your nose and tongue work to distinguish the difference between the smells and tastes of spearmint or caraway or dill through specialized receptors that interact with molecules in very specific ways.
Think of some receptors as structured like baseball mitts specifically designed to pick up molecules with structures like baseballs but not footballs which have their own receivers. These receptors are so highly specialized that they interact distinctively with molecules that differ only in very small ways, like their handedness, almost as if some receptors are like right-handed gloves able to pick up only the R-carvone molecules, recognize them, and send a message to the brain saying Its spearmint!
And as if S-carvones only fit into left-handed glove receptors, who recognize them and tell the brain: Its caraway! or Its dill!
There you go: a handy explanation of carvone.
學習單
國中:Molecules, Isomers, and Our World
高中:Introduction to Enantiomers and Handedness
《上一單元:吉士堡化學--乳酪 ‖ 下一單元:化學鍵》
進一步參考資料
1. NBC Learn- Chemistry now
2. NSTA Blog
旁白:
"Mirror" Molecule: Carvone
Spearmint. Caraway. Dill.
A chewing gum flavor. A seed in rye bread. And an herb in pickles. Wouldnt seem they have anything in common, but they do. Reduce spearmint, caraway seed and dill to their essential oils and a sizable percentage of all three turn out to be made up of the same molecule: carvone.
How can you explain one molecule being responsible for distinctly different smells and tastes? With two hands, a mirror, a lightbulb and a pair of gloves.
Start with the basics: Carvones chemical formula tells you what its made of: 10 atoms of Carbon, 14 atoms of Hydrogen, and 1 atom of Oxygen. Just as important as how many atoms of what elements make up a molecule is how those atoms are bonded together and in what configuration, or structure.
We think of molecules, when and if we think of molecules, as having only one set structure. This is H2O, for example not this, or this. But a molecule like Carvone can have slightly different arrangements of their atoms and still be the same molecule like a girl who has different looks depending on how she parts her hair, or the way she wears a sweater. As long as how she rearranges her hair and clothes doesnt add or take away anything, shes still the same girl in the same outfit.
The carvone molecule has two slightly different looks or, as theyre called, stereoisomers: this one and this one. Stereoisomers are three-dimensional, but its easier to understand these two by looking at a standard two-dimensional drawing of their structures. Notice anything? The two are mirror images of each other. Some structures, like, say, a lightbulb, are the same in mirror image or side by side. But carvone stereoisomers are like a pair of hands: A hand and its mirror reflection will exactly match but hands arent the same side by side, or superimposed.
Actually, carvone stereoisomers are like left and right hands even down to their names: This one is R-carvone the R stands for the Latin word meaning right. The structure of this gives spearmint its taste and smell. Its mirror opposite is S-carvone the S stands for the Latin word meaning left. The way this one is arranged is what gives caraway its flavor and aroma and dill, too. In pure form, the two flavors are almost the same.
This kind of left-handed/right-handed molecule is called chiral. Carvone has chirality, terms, not coincidentally, from a Greek word meaning hand. The Greek word for nose is rhinous, yes, as in rhinocerous. which is the way well segue into this next part on how your nose and tongue work to distinguish the difference between the smells and tastes of spearmint or caraway or dill through specialized receptors that interact with molecules in very specific ways.
Think of some receptors as structured like baseball mitts specifically designed to pick up molecules with structures like baseballs but not footballs which have their own receivers. These receptors are so highly specialized that they interact distinctively with molecules that differ only in very small ways, like their handedness, almost as if some receptors are like right-handed gloves able to pick up only the R-carvone molecules, recognize them, and send a message to the brain saying Its spearmint!
And as if S-carvones only fit into left-handed glove receptors, who recognize them and tell the brain: Its caraway! or Its dill!
There you go: a handy explanation of carvone.
學習單
國中:Molecules, Isomers, and Our World
高中:Introduction to Enantiomers and Handedness
《上一單元:吉士堡化學--乳酪 ‖ 下一單元:化學鍵》
進一步參考資料
1. NBC Learn- Chemistry now
2. NSTA Blog
今日化學 :吉士堡化學—乳酪
Chemistry Now : Cheeseburger Chemistry—Cheese
美國科學基金會(NSF)、美國科學教師協會(NSTA)及美國國家廣播公司(NBC)合作,為化學年製作了一系列教學單元,名為「今日化學(Chemistry Now)」,每周推出一單元,預計製作31單元。內容涵蓋日常生活中的化學,包含了21世界的先進化學。以下是第二單元:吉士堡化學--乳酪(Chemistry of Cheeseburger--Cheeses)。
旁白:
The Chemistry of Cheese
AL ROKER, reporting:
You cant have a cheeseburger without it: cheese. Cheddar, Swiss, Mozzarella, Blue, Monterrey jack, Pepperjack. To keep the chemistry more basic, we wont deal here with whats called American or processed cheese.
Cheese is an ancient food, dating back some 4,000 years to when humans first domesticated goats, sheep, yaks and other mammals for meat and milk: the sole basic ingredient in cheese, then and today.
JULIE YU, The Exploratorium: Cheese is a very concentrated form of milk with the water removed.
ROKER: Turning milk into cheese involves a change in a substance from one common state of matter to another, in this case, from liquid to solid. Some of these changes can be physical those are changes that are reversible, like freezing water into a solid ice cube: it can melt into water again.
When a change involves a chemical reaction, it generally cant be reversed and turning liquid milk to solid cheese is a good example: the cheese can never go back to being milk again. Heres why:
YU: Were going to make the worlds simplest cheese.
ROKER: Julie Yu, a scientist at The Exploratorium in San Francisco whos funded by The National Science Foundation, starts with milk.
YU: Milk is composed of proteins, fats, sugars and water. The process of making cheese is somehow removing that water so that youre left with the concentrated mass of the proteins and fats.
ROKER: Thats not so easy. Milk is an emulsion: uncounted illions of globular protein molecules and droplets of fat are suspended in the liquid. How to separate those from the water, and concentrate them? Think of panning for gold. If the gold was in the form of tiny individual flecks, itd be impossible to pan out; itd just flow through any strainer with the water. The gold has to be in clumps, nuggets, to be sifted out. So how do you get the fats and proteins in liquid milk to form little nuggets so they can be separated from the water?
YU: Cheesemaking relies on changing the structure of the proteins that are in milk because in order to separate out the proteins from the water in our milk, we need to change their form.
ROKER: Its called denaturing, which is pretty much what it sounds like: changing the natural structure or qualities of something.
YU: Proteins are typically folded up in a three-dimensional structure. When theyre denatured, they relax into a long chain. And so those chains can tangle together and become enmeshed and they solidify in a way that you are able to strain them from the milk. In general, there are three ways to denature proteins: one is to introduce heat, one is to introduce high salt, and one is to introduce acid.
ROKER: Like the citric acid in lemon juice.
YU: Were going to use lemon juice today. The proteins are normally in tight little balls. The acid is going to relax them. And theyre going to coagulate, theyre going to stick together in this nice gooey mess. And were going to strain that out and that will give us the cheese.
Im going to pour this through a strainer that I have lined with some cheesecloth. And thats going to keep the solids behind, which we now call the curds. And the liquid, which we would call the whey, is going into this bowl.
ROKER: Yes, curds and whey, what Little Miss Muffet was eating, as she sat on whatever a tuffet is, before unexpected arachnid proximity prompted flight.
YU: Once we strain out all of the whey it firms up into this nice ball of fresh cheese. There are some fresh cheeses that are made this way, just by simply adding acid. But the majority of cheeses are made in another way. They use a bacteria and an enzyme in order to coagulate their proteins.
ROKER: An enzyme called rennet, which cheesemakers can buy in tablet form.
YU: Rennet is an enzyme thats actually in the stomach lining of most animals. Its made to digest milk proteins. Rennet further breaks down the proteins and creates this nice gooey mesh and gives you cheeses of different textures.
ROKER: Rennet may explain how those ancient ancestors of ours made the first cheese.
YU: Its possible that someone had a pouch made out of animal stomach and was holding milk inside of that. Any enzymes present in the stomach would break down the milk and when they poured out their milk they would have been surprised to find curds and whey.
ROKER: Today cheeses come in a global array - at least 670 different kinds are listed in a leading cheese database. Chemistry is the reason all those different textures and flavors develop during cheese processing and aging: fermentation, oxidation, dehydration, bacterial and mold growth. Theyre all chemical reactions. So, there you are: a basic explanation of the chemical processes that turn liquid milk into solid cheese and turn a hamburger into a cheeseburger.
學習單
國中:Blowing up balloons with yeast
高中:Kitchen Mystery
《上一單元:水的化學 ‖ 下一單元:分子結構》
進一步參考資料
1. NBC Learn- Chemistry now
2. NSTA Blog
旁白:
The Chemistry of Cheese
AL ROKER, reporting:
You cant have a cheeseburger without it: cheese. Cheddar, Swiss, Mozzarella, Blue, Monterrey jack, Pepperjack. To keep the chemistry more basic, we wont deal here with whats called American or processed cheese.
Cheese is an ancient food, dating back some 4,000 years to when humans first domesticated goats, sheep, yaks and other mammals for meat and milk: the sole basic ingredient in cheese, then and today.
JULIE YU, The Exploratorium: Cheese is a very concentrated form of milk with the water removed.
ROKER: Turning milk into cheese involves a change in a substance from one common state of matter to another, in this case, from liquid to solid. Some of these changes can be physical those are changes that are reversible, like freezing water into a solid ice cube: it can melt into water again.
When a change involves a chemical reaction, it generally cant be reversed and turning liquid milk to solid cheese is a good example: the cheese can never go back to being milk again. Heres why:
YU: Were going to make the worlds simplest cheese.
ROKER: Julie Yu, a scientist at The Exploratorium in San Francisco whos funded by The National Science Foundation, starts with milk.
YU: Milk is composed of proteins, fats, sugars and water. The process of making cheese is somehow removing that water so that youre left with the concentrated mass of the proteins and fats.
ROKER: Thats not so easy. Milk is an emulsion: uncounted illions of globular protein molecules and droplets of fat are suspended in the liquid. How to separate those from the water, and concentrate them? Think of panning for gold. If the gold was in the form of tiny individual flecks, itd be impossible to pan out; itd just flow through any strainer with the water. The gold has to be in clumps, nuggets, to be sifted out. So how do you get the fats and proteins in liquid milk to form little nuggets so they can be separated from the water?
YU: Cheesemaking relies on changing the structure of the proteins that are in milk because in order to separate out the proteins from the water in our milk, we need to change their form.
ROKER: Its called denaturing, which is pretty much what it sounds like: changing the natural structure or qualities of something.
YU: Proteins are typically folded up in a three-dimensional structure. When theyre denatured, they relax into a long chain. And so those chains can tangle together and become enmeshed and they solidify in a way that you are able to strain them from the milk. In general, there are three ways to denature proteins: one is to introduce heat, one is to introduce high salt, and one is to introduce acid.
ROKER: Like the citric acid in lemon juice.
YU: Were going to use lemon juice today. The proteins are normally in tight little balls. The acid is going to relax them. And theyre going to coagulate, theyre going to stick together in this nice gooey mess. And were going to strain that out and that will give us the cheese.
Im going to pour this through a strainer that I have lined with some cheesecloth. And thats going to keep the solids behind, which we now call the curds. And the liquid, which we would call the whey, is going into this bowl.
ROKER: Yes, curds and whey, what Little Miss Muffet was eating, as she sat on whatever a tuffet is, before unexpected arachnid proximity prompted flight.
YU: Once we strain out all of the whey it firms up into this nice ball of fresh cheese. There are some fresh cheeses that are made this way, just by simply adding acid. But the majority of cheeses are made in another way. They use a bacteria and an enzyme in order to coagulate their proteins.
ROKER: An enzyme called rennet, which cheesemakers can buy in tablet form.
YU: Rennet is an enzyme thats actually in the stomach lining of most animals. Its made to digest milk proteins. Rennet further breaks down the proteins and creates this nice gooey mesh and gives you cheeses of different textures.
ROKER: Rennet may explain how those ancient ancestors of ours made the first cheese.
YU: Its possible that someone had a pouch made out of animal stomach and was holding milk inside of that. Any enzymes present in the stomach would break down the milk and when they poured out their milk they would have been surprised to find curds and whey.
ROKER: Today cheeses come in a global array - at least 670 different kinds are listed in a leading cheese database. Chemistry is the reason all those different textures and flavors develop during cheese processing and aging: fermentation, oxidation, dehydration, bacterial and mold growth. Theyre all chemical reactions. So, there you are: a basic explanation of the chemical processes that turn liquid milk into solid cheese and turn a hamburger into a cheeseburger.
學習單
國中:Blowing up balloons with yeast
高中:Kitchen Mystery
《上一單元:水的化學 ‖ 下一單元:分子結構》
進一步參考資料
1. NBC Learn- Chemistry now
2. NSTA Blog
今日化學 :水的化學
Chemistry Now : Chemistry of Water
美國科學基金會(NSF)、美國科學教師協會(NSTA)及美國國家廣播公司(NBC)合作,為化學年製作了一系列教學單元,名為「今日化學(Chemistry Now)」,每周推出一單元,預計製作31單元。內容涵蓋日常生活中的化學,包含了21世界的先進化學。以下是第一單元:水的化學(Chemistry of Water)。
旁白:
Chemistry Now: Molecule Profile: H20 - Water
It might just be the most universally known fact in chemistry: the chemical formula for water - H2O.
A model of H2O doesnt look like much two small atoms of Hydrogen, the H2 part of H2O, attached to one bigger atom of Oxygen, the O part of H2O. Kind of like a cartoon drawing of a teddy bear and actually, water is kind of funny.
Fun Facts: Water H2O is the only natural substance on Earth found in all three common states of matter: liquid, solid, and gas, or vapor.
Its also one of the only common substances that is less dense in solid form than in liquid form. And water can dissolve more substances than any other liquid.
Lets focus on H2O in liquid form: What gives water its remarkable qualities and abilities?
Its all in the molecules content and structure not just what each H2O molecule is made of, but how the atoms are positioned, in what configuration and shape, and how they are bound together.
In water, just so youll know for later, the Hs and O are held together by covalent bonds by sharing electrons.
H2O is a polar molecule: The same way our planet has North and South Poles on opposite sides, the H2O molecule has two poles on opposite sides. And like a magnet, H2O has one positive end and one negative end.
Thinking of that cartoon teddy bear again, the chin side of the Oxygen atom has a slight negative electrical charge; the opposite side the side with the two Hydrogen ears has a slight positive electrical charge.
This might just be the second most universally known bit of chemistry: opposites attract. The positive Hydrogen side of every H2O molecule is going to attract, and be attracted to, the negative Oxygen sides of other nearby H2O molecules, in all directions. More and more of them pull closer and closer together until theyre like people in a hot, crowded dance club, packed so close together they can hardly move, but still turning and moving wildly.
Molecules that stay close to each other are called cohesive and water is highly cohesive. A pentillion even hextillion of chaotically-moving, tightly-packed H2O molecules cohere to make a single raindrop.
As good as water molecules are at all this cohering and convening, they also form bonds with surfaces and molecules unlike themselves a force called adhesion, as in adhesive. Pour the water out of a glass, and the inside of the glass is still wet: some H2O molecules stick or adhere to the silica molecules in the glass.
Being cohesive and adhesive is what makes water a (near) universal solvent. How? Take salt, or sodium chloride sodium ions, abbreviated as Na-plus, and chloride ions, abbreviated as Cl-minus, in a crystal.
As soon as a salt crystal hits the water, its rushed by a molecular mob of H2O molecules that break it apart; separate the sodium and chloride ions kind of a chemical divide and conquer.
Because sodium ions have a positive charge, each one is swarmed by H2O molecules flying at it with their negatively-charged Oxygen sides, completely surrounding the sodium ion, isolating it, carrying it off in a turbulent sea of H2O molecules.
The same thing happens in reverse to the chloride ions, which have a negative charge each of those is surrounded and isolated by H2O molecules leading with their positively-charged hydrogen sides.
Each salt crystal is broken into tiny pieces dissolved by, and into, the water.
Theres much, much more to know about H2O and how it works to keep every living thing on the planet and maybe other planets alive.
Think of this video as a drop in the bucket.
學習單
國中:Density Comparison of Water and Ice
高中:Water is a Polar Molecule
‖ 下一單元:吉士堡化學--乳酪》
進一步參考資料:
1. NBC Learn- Chemistry now
2. NSTA Blog
旁白:
Chemistry Now: Molecule Profile: H20 - Water
It might just be the most universally known fact in chemistry: the chemical formula for water - H2O.
A model of H2O doesnt look like much two small atoms of Hydrogen, the H2 part of H2O, attached to one bigger atom of Oxygen, the O part of H2O. Kind of like a cartoon drawing of a teddy bear and actually, water is kind of funny.
Fun Facts: Water H2O is the only natural substance on Earth found in all three common states of matter: liquid, solid, and gas, or vapor.
Its also one of the only common substances that is less dense in solid form than in liquid form. And water can dissolve more substances than any other liquid.
Lets focus on H2O in liquid form: What gives water its remarkable qualities and abilities?
Its all in the molecules content and structure not just what each H2O molecule is made of, but how the atoms are positioned, in what configuration and shape, and how they are bound together.
In water, just so youll know for later, the Hs and O are held together by covalent bonds by sharing electrons.
H2O is a polar molecule: The same way our planet has North and South Poles on opposite sides, the H2O molecule has two poles on opposite sides. And like a magnet, H2O has one positive end and one negative end.
Thinking of that cartoon teddy bear again, the chin side of the Oxygen atom has a slight negative electrical charge; the opposite side the side with the two Hydrogen ears has a slight positive electrical charge.
This might just be the second most universally known bit of chemistry: opposites attract. The positive Hydrogen side of every H2O molecule is going to attract, and be attracted to, the negative Oxygen sides of other nearby H2O molecules, in all directions. More and more of them pull closer and closer together until theyre like people in a hot, crowded dance club, packed so close together they can hardly move, but still turning and moving wildly.
Molecules that stay close to each other are called cohesive and water is highly cohesive. A pentillion even hextillion of chaotically-moving, tightly-packed H2O molecules cohere to make a single raindrop.
As good as water molecules are at all this cohering and convening, they also form bonds with surfaces and molecules unlike themselves a force called adhesion, as in adhesive. Pour the water out of a glass, and the inside of the glass is still wet: some H2O molecules stick or adhere to the silica molecules in the glass.
Being cohesive and adhesive is what makes water a (near) universal solvent. How? Take salt, or sodium chloride sodium ions, abbreviated as Na-plus, and chloride ions, abbreviated as Cl-minus, in a crystal.
As soon as a salt crystal hits the water, its rushed by a molecular mob of H2O molecules that break it apart; separate the sodium and chloride ions kind of a chemical divide and conquer.
Because sodium ions have a positive charge, each one is swarmed by H2O molecules flying at it with their negatively-charged Oxygen sides, completely surrounding the sodium ion, isolating it, carrying it off in a turbulent sea of H2O molecules.
The same thing happens in reverse to the chloride ions, which have a negative charge each of those is surrounded and isolated by H2O molecules leading with their positively-charged hydrogen sides.
Each salt crystal is broken into tiny pieces dissolved by, and into, the water.
Theres much, much more to know about H2O and how it works to keep every living thing on the planet and maybe other planets alive.
Think of this video as a drop in the bucket.
學習單
國中:Density Comparison of Water and Ice
高中:Water is a Polar Molecule
‖ 下一單元:吉士堡化學--乳酪》
進一步參考資料:
1. NBC Learn- Chemistry now
2. NSTA Blog
2011/03/28
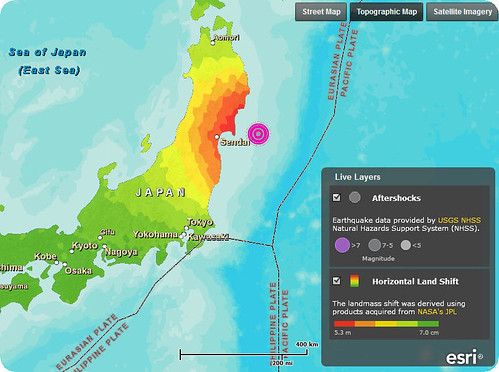
教學時機:為何311日本大地震使地球自轉輕微變快?
日本地震提供了一個教學機會,NASA的太空數學Space Math就提供了一些題目,可供教師教學運用。
新聞報導中一直強調地震使得地球自轉變快,太空數學就運用簡單的物理模式,讓學生探索日本地震使地球自轉變快1.8微秒的原理。教師可運用在角動量守恆的教學上。
以下是題目(The 2011 Japan Earthquake Rocks the Earth)的中文翻譯。
根據在加州的美國太空總署噴射推進實驗室的地球物理學家Richard Gross的計算,2011年3月11日的日本地震導致地球自轉變快約1.8微秒(0.0000018秒)。這看來很小,但是在今日高科技的世界裡,時間通常以十億分之一秒計,這就是非常巨大的改變!
導致地球自轉變快的原因類似於旋轉的溜冰者在旋轉時縮回她的手臂。手臂的質量越靠近她的旋轉軸會導致她旋轉越快以維持角動量的守恆。
地震可能移動質量,像日本島,使其稍微靠近地球中心,因此地球旋轉變快以維持角動量守恆。日本移動了將近4公尺,這顯著地改變了物質在地殼(就像溜冰者的手)的分布方式。這是怎麼發生的? 底下是一個簡單的模型。
問題1 — 球體的角動量公式如下:
如果質量M不變,角動量J也不變,哪一個公式可以用來表示最初的半徑r和角速度ω對最後的半徑和角速度的比較?
問題2 — 如果地球在整整24小時,以半徑6378.00km轉了2π的角度,在它內縮1.00公里後,最後的角速度變為多少rad/s?
問題3 — 地球自轉週期的差為多少秒?
當然,日本地震的地殼移動型式比本問題更為複雜,但是基本原理一樣。
相關文章及資料:
新聞報導中一直強調地震使得地球自轉變快,太空數學就運用簡單的物理模式,讓學生探索日本地震使地球自轉變快1.8微秒的原理。教師可運用在角動量守恆的教學上。
以下是題目(The 2011 Japan Earthquake Rocks the Earth)的中文翻譯。
2011日本地震撼動地球
根據在加州的美國太空總署噴射推進實驗室的地球物理學家Richard Gross的計算,2011年3月11日的日本地震導致地球自轉變快約1.8微秒(0.0000018秒)。這看來很小,但是在今日高科技的世界裡,時間通常以十億分之一秒計,這就是非常巨大的改變!
導致地球自轉變快的原因類似於旋轉的溜冰者在旋轉時縮回她的手臂。手臂的質量越靠近她的旋轉軸會導致她旋轉越快以維持角動量的守恆。
冬季奧運花式溜冰的角動量守恆
地震可能移動質量,像日本島,使其稍微靠近地球中心,因此地球旋轉變快以維持角動量守恆。日本移動了將近4公尺,這顯著地改變了物質在地殼(就像溜冰者的手)的分布方式。這是怎麼發生的? 底下是一個簡單的模型。
問題1 — 球體的角動量公式如下:
如果質量M不變,角動量J也不變,哪一個公式可以用來表示最初的半徑r和角速度ω對最後的半徑和角速度的比較?
問題2 — 如果地球在整整24小時,以半徑6378.00km轉了2π的角度,在它內縮1.00公里後,最後的角速度變為多少rad/s?
問題3 — 地球自轉週期的差為多少秒?
當然,日本地震的地殼移動型式比本問題更為複雜,但是基本原理一樣。
相關文章及資料:
- 大地震帶來大迷思! 大地震會造成地球自轉軸偏移嗎?
- 冬季奧運花式溜冰的角動量守恆(教學活動設計,評量)
- 本問題PDF檔及參考答案(英文,中文)
2011/03/22
美國化學學會提供免費教學資源
美國化學學會(The American Chemical Society, ACS)建立一個教學資源網站,提供以活動為基礎的中學化學教學設計。教學單元含蓋中學化學的所有主要概念,並配有動畫或影片。網址如下:
http://www.middleschoolchemistry.com/
大地震帶來大迷思! 大地震會造成地球自轉軸偏移嗎?
以下摘自中國時報的報導:
日本「地理網」(Geonet)蒐集的資料顯示,三月十一日的東日本大地震,導致日本海岸向東位移最多達四公尺,並導致地軸偏移約十六.五公分,從而稍微加快地球自轉的速度,地球一天的長度也將因此縮短一百八十萬分之一秒(這應該是翻譯錯誤,應該是1.8微秒,即1.8百萬分之一秒,或百萬分之1.8秒)。(中國時報, 2011-03-16)
這個地軸是指地球自轉軸嗎?
許多新聞報導都直指地球自轉軸! 在網路上以「地球自轉軸偏移」做關鍵字搜尋,可以搜尋到二萬則左右的資料。甚至也有學者據此推論「希望地球的自轉軸別偏太多了,雖然磁軸已偏太多了。地上有海嘯,但希望地下的"岩漿嘯"別震盪太大,一旦自轉軸偏太大,可能要改變公轉軌道了,這"代誌"就大了!」有醬恐怖嗎?
但是根據物理定律(角動量守恆):轉動的物體如果沒有外來的力(嚴格來說,應該說沒有外力矩)作用,轉動(或轉動軸)的狀態就不會改變,亦即角動量的大小和方向維持不變。所以這裡的地軸變動不應該是地球自轉軸指向太空的方向變動! 如果真是這個變動,那就真的代誌大條,物理定律要改寫了!
底下摘錄自聯合報記者在智利大地震後訪問台大地質學系洪教授的報導:
「地軸偏移」、「自轉變快」,究竟稀不稀奇?台灣大學地質科學系副教授洪淑蕙指出,這並不算很「炫」的事,因為平常的風能和潮汐,造成地球每天延長或縮短的時間,就超過智利地震的影響千倍以上。更何況「1.26微秒」根本沒人感覺得出來。
洪淑蕙解釋,所謂「地球自轉」可想像地球繞著穿過質心的一條假想軸-「質心軸」轉動,智利地震的成因是納斯卡板塊隱沒於南美洲板塊,讓整個球體的質量分布稍微改變,所以造成質心和質心軸些許移動。
至於地球轉動變快,是因為智利地震與「板塊隱沒」有關,可以想像成納斯卡板塊略為「往裡縮」,讓地球整體的轉動慣量變小。但是,地震算是地球自身的「內力」作用,沒有外力干擾,根據角動量守恆定律,地球的角速度變大,轉得快了一些。(聯合報, 2010-4-5)
這樣看來聯合報的報導裡,地軸指的是「質心軸」。問題是:質心軸是否和自轉軸重疊?
美國太空總署(NASA)的地球物理學家Richard Gross這樣說:
The Earth rotates around its rotation axis, but its mass is balanced about a different axis, the figure axis. Because these axes are different, the Earth wobbles as it rotates.(Beitler, 2010, 摘錄自NASA網頁)
地球繞著它的自轉軸旋轉,但是它的質量卻是對另一個不同的軸--質心軸(註1)平衡。因為這兩個軸不同,所以地球自轉時會晃動。
也就是說,地球像中心未對準轉軸而鎖歪了的偏心汽車輪子,當輪子轉動時會有晃動現象。
我們再來看看美國太空總署(NASA)的新聞稿怎麼說?
The calculations also show the Japan quake should have shifted the position of Earth's figure axis (the axis about which Earth's mass is balanced) by about 17 centimeters (6.5 inches), towards 133 degrees east longitude. Earth's figure axis should not be confused with its north-south axis; they are offset by about 10 meters (about 33 feet). This shift in Earth's figure axis will cause Earth to wobble a bit differently as it rotates, but it will not cause a shift of Earth's axis in space—only external forces such as the gravitational attraction of the sun, moon and planets can do that.(NASA, 2011-3-14)
計算同時顯示,日本地震應該已經使得地球質心軸(地球質量對此軸平衡)的位置向東經133度偏移了大約17公分。地球質心軸不應該和地球南北軸(即地球在太空中的自轉軸,註2)混淆,兩者大約相距10公尺。這次地球質心軸的偏移將使得地球自轉時的晃動(註3)和以往有一點點不同,但是它不會造成太空中的地軸變動(指自轉軸指向的變動)——只有外力(如太陽、月亮或行星的萬有引力)才能造成這種變動。
原來地球指向天球的自轉軸並沒有變動,偏移的是質心軸。大地震改變了地球的質量分佈,所以造成質心軸變動,就像汽車輪子撞到大坑洞,結果輪子變了形,它的質心移了位置。但是地震不是外來的力,所以自轉軸相對於天球沒有變動。
謝天謝地! 物理定律終究不用改寫!
這個誤會(註4)讓我們學到以下幾點:
- 報紙或網路上的資訊不能完全相信,所以我們必須學會並教學生如何分析、判斷及解讀資訊的可信程度。
- 不只是學生會有迷思概念,其實每個人都有迷思概念,即使是學者專家,在他非專長的領域,也和普通人一樣具有迷思概念。所以,我們應該學習尊重真正具有該領域專長的專家學者意見,而不是輕易相信所謂專家學者的意見,也許這些專家學者的專長並不在此。
- 如果提供的資訊越充足,產生迷思的可能性就越低,因此報紙不應該刪除前面所引用NASA新聞稿的那一段。老師的教學也是。如果洪淑蕙教授能再提一下,或記者能夠追問,真實地球的質心軸和南北自轉軸是否不同,那麼產生迷思的機會可能會降低。
- 我們終於知道「地軸(Earth's axis)」這兩個字有可能指質心軸(figure axis)或南北軸(north-south axis)。附帶一提,這裡的南北是指自轉軸上的南北,不是磁針所指的地磁南北,這兩者也是不同的。
- 高中老師在以後講解角動量守恆時,可以舉「大地震可能造成地球自轉變快(因地球內縮,轉動慣量變小,而角動量必須守恆,所以轉動變快),但不會造成相對於天球的自轉軸方向變動(因無外力作用,所以無外力矩作用,故地球相對於天球的自轉軸不會改變)」這個活生生的例子。
註1:
figure axis, 直譯是形狀軸,意譯也許也可以翻譯成平衡軸,本文根據台大洪教授的用詞,將其翻譯為質心軸。
註2:
Space.com根據NASA地球物理學家Richard Gross對日本地震對地球影響的研究報導中,對南北軸(north-south axis)及質心軸(figure axis)的解釋如下:
The Earth's figure axis is not the same as its north-south axis in space, which it spins around once every day at a speed of about 1,000 mph (1,674 kph). The figure axis is the axis around which the Earth's mass is balanced and the north-south axis by about 33 feet (10 meters).(Space.com, 2011-3-13)
地球的質心軸和它在太空中的南北軸不同。地球每天以大約每小時1000英哩(1674公里/小時)的速率繞南北軸一次。質心軸是指地球質量對其平衡的軸,在南北軸旁邊約33英呎(10公尺)。
註3:
如果對地球自轉的晃動現象(錢德勒晃動Chandler Wobble)有興趣,可參考下列網頁:
http://en.wikipedia.org/wiki/Chandler_wobble
http://nasadaacs.eos.nasa.gov/articles/2010/2010_gps.html
註4:
地球自轉軸在太空中沒有變動,晃動的是質心軸;相對來說,我們可以看到自轉軸的極點在地球上移動,叫做polar motion(極移)。大地震發生後,實際是質心軸極輕微偏移,相對而言,我們看到地球自轉軸在地球上的相對位置有極輕微偏移,所以實際上有物理性質變動的不是自轉軸,而是質心軸。
 |
| 2000-2009年地球自轉軸的極點在地球上移動 |
2011/03/11
體育活動的科學
美國國家科學基金會與國家廣播公司合作拍攝了三套體育活動相關的科學影片,利用體育活動來教科學。
1. 美式足球的科學 Science of NFL Football
http://www.nsf.gov/news/special_reports/football/index.jsp
2. 冬季奧運的科學 Science of the Olympic Winter Games
http://www.nsf.gov/news/special_reports/olympics/index.jsp
3. 賽車的科學 The Science of Speed
http://www.nsf.gov/news/special_reports/sos/
影片雖然是英語發音,不過美式足球的科學可以點選影片視窗上的[CC]按鈕,將其切換成On,就會出現英文字幕。
每部影片的長度約在4-5分鐘,並提供教學計畫供老師參考,很適合在教室中配合教學使用。
1. 美式足球的科學 Science of NFL Football
http://www.nsf.gov/news/special_reports/football/index.jsp
2. 冬季奧運的科學 Science of the Olympic Winter Games
http://www.nsf.gov/news/special_reports/olympics/index.jsp
3. 賽車的科學 The Science of Speed
http://www.nsf.gov/news/special_reports/sos/
影片雖然是英語發音,不過美式足球的科學可以點選影片視窗上的[CC]按鈕,將其切換成On,就會出現英文字幕。
每部影片的長度約在4-5分鐘,並提供教學計畫供老師參考,很適合在教室中配合教學使用。
2011/02/05
介紹三本科學教育書籍
Taking Science to School: Learning and Teaching Science in Grades K-8
Ready, Set, SCIENCE!: Putting Research to Work in K-8 Science Classrooms
Surrounded by Science: Learning Science in Informal Environments
Ready, Set, SCIENCE!: Putting Research to Work in K-8 Science Classrooms
Surrounded by Science: Learning Science in Informal Environments
2011/01/19
Google舉辦全球網上科學展覽競賽
Google科學展覽會( The Google Science Fair)
即日起線上報名。最後入選者將於2011年7月,在加州Google總部,面對評審小組(包括諾貝爾獎得主及著名科學家)競逐實習機會、獎學金、及獎項。
競賽網址:
http://www.google.com/events/sciencefair/
人:參加者為全球13-18歲學生,個人或最多3人的團隊。
事:全球性科學、技術、工程、及數學競賽。
時:競賽截止日2011年4月4日。
地:註冊及作品繳交均在網路上。
物:相關資料可從下列網址下載
http://www.google.com/events/sciencefair/teachers.html
主辦:
Google
協辦:
美國太空總署(NASA)、歐洲核子研究委員會(CERN)、國家地理雜誌(National Geographic)、科學美國人(Scientific American)、以及樂高集團( LEGO Group)
即日起線上報名。最後入選者將於2011年7月,在加州Google總部,面對評審小組(包括諾貝爾獎得主及著名科學家)競逐實習機會、獎學金、及獎項。
競賽網址:
http://www.google.com/events/sciencefair/
人:參加者為全球13-18歲學生,個人或最多3人的團隊。
事:全球性科學、技術、工程、及數學競賽。
時:競賽截止日2011年4月4日。
地:註冊及作品繳交均在網路上。
物:相關資料可從下列網址下載
http://www.google.com/events/sciencefair/teachers.html
主辦:
協辦:
美國太空總署(NASA)、歐洲核子研究委員會(CERN)、國家地理雜誌(National Geographic)、科學美國人(Scientific American)、以及樂高集團( LEGO Group)
2011/01/12
2011國際化學年
2011是國際化學年(International Year of Chemistry 2011),一整年將舉辦許多精采的活動,並提供豐富的教學資源。
相關網頁如下:
IYC2011 官方網站:
http://www.chemistry2011.org/
IYC2011 台灣官方網站:
http://iyc2011.tku.edu.tw/
相關資源:
The Periodic Table of Videos
除提供週期表118個元素的118部影片外,還有分子影片、化學之旅及其他影片。影片為英語發音,可選擇顯示英文字幕。
http://www.periodicvideos.com/
魔幻化境Magichem
提供有趣的化學知識及實驗影片,以及台灣的化學年活動資訊。
http://case.ntu.edu.tw/magichem/blog/
訂閱:
文章 (Atom)